Protocol - Refractive Error - Adult
Description
The refractive error of each eye is measured using the Humphrey Automated Refractor (Carl Zeiss Meditec, Dublin, CA). After use of the autorefractor, the autorefractor-generated refractive error for each eye is dialed in to the phoropter and the participants visual acuity is checked. If the visual acuity is worse than 20/20, then subjective refraction is performed.
Specific Instructions
This protocol uses the Humphrey Automated Refractor (Carl Zeiss Meditec, Dublin, CA). Other instruments can be used to measure refractive error. If other instruments are used, the reproducibility of the measurements should be comparable to those acquired with this protocol. In addition, when other instruments are used to collect these measurements, the manufacturer and model of equipment should be recorded. These other devices may require some different steps than are described in this protocol. Investigators should follow the equipment manufacturers instructions to ensure quality control.
Availability
Protocol
Only participants who read less than the 20/20 line at presenting monocular visual acuity will be refracted with the Humphrey Automated Refractor.
Procedure for Humphrey Automated Refraction
1. Wipe participant chin rest and headrest with alcohol swab.
2. Turn on the instrument; you will automatically be in the mode to take a reading.
3. Adjust the height of the chin by rotating the chin rest adjustment ring so that the outer canthus of the participant is in alignment with the marker on the side of the machine.
4. You should now see the participants eye on the screen. Looking at the TV monitor, center the iris in the ring on the screen.
5. Press the measurement button on the joystick.
6. Repeat procedure on left eye.
7. Press PRINT.
8. If the participant can read 20/20 on the autorefractor, place the appropriate prescription for each eye in the phoropter.
9. Ask the participant to read all the letters on Chart 1 with the right eye. Encourage the participant to try to read each smaller line on the chart to ensure that s/he makes a maximal effort with each eye.
10. When the participant cannot read a letter, encourage him/her to guess. If the participant states that a letter is one of two letters, ask him/her to choose only one letter and, if necessary, to guess.
11. Record the number of letters read correctly for each line in the computer. The computer will then calculate the visual acuity for the participant.
12. Repeat the same procedure for the left eye with Chart 2.
13. If the visual acuity is worse than 20/20, perform subjective refraction; if it is 20/20 or better, the participant has completed refraction.
Subjective Refraction
Procedure for determining the sphere
1. Using the results from the automated refractor, ask the participant to focus on one line above the lowest line read correctly as a reference point.
2. Add +0.50 sphere and ask the participant, "Does it make the letters better, worse or the same?" Continue to add plus sphere until the participant responds that it makes his/her vision worse. This process determines the highest plus (or least minus) sphere that the participant will accept.
3. Next, present a-0.25 diopter sphere, again asking "Does this lens make the letters better, worse or the same or smaller and darker?" Each time the participants response is "better," s/he must be able to demonstrate improvement by reading additional letters. If the participant is unable to read additional letters, the amount of spherical change (-0.25 diopters) will be removed.
Cylinder axis determination
1. Changes in cylindrical axis are determined by straddling the starting point axis with a 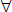 0.25 diopter cross-cylinder.
0.25 diopter cross-cylinder.
2. Ask the participant to look at a round letter on the lowest line read.
3. If the participant prefers one position of the cross-cylinder to the other, the axis of the cylinder is moved 5 degrees toward the positive axis of the cross cylinder. Once the participant feels that the two choices are the same, the technician will again confirm the preferred axis is correct. Testing for a change of axis is repeated until the participant finds both positions of the cross-cylinder the same.
Cylinder power determination
1. If no cylinder is initially present, place the 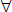 0.25 diopter Jackson cross-cylinder with the positive axis first at 90 degrees and at 180 degrees, then 45 degrees and 135 degrees while having the participant look at a round letter 2 lines above the previous MVA line. If the participant states that the vision is improved at any one of these four axis positions, place a +0.25 D cylindrical lens in the phoropter at the preferred axis and continue to refine the axis, as described above.
0.25 diopter Jackson cross-cylinder with the positive axis first at 90 degrees and at 180 degrees, then 45 degrees and 135 degrees while having the participant look at a round letter 2 lines above the previous MVA line. If the participant states that the vision is improved at any one of these four axis positions, place a +0.25 D cylindrical lens in the phoropter at the preferred axis and continue to refine the axis, as described above.
2. Following axis refinement, changes in cylindrical power are determined by aligning the power of the initial cylinder with the axes of the cross-cylinder lens.
3. If the participant prefers the positive meridian, increase the cylindrical power by + 0.25 diopters.
4. If the participant prefers the negative meridian, decrease the cylindrical power by - 0.25 diopters.
5. The process is repeated until the participant detects no difference in clarity between the two positions of the cross-cylinder lens.
6. For each +0.50 diopter of cylindrical power added, the spherical power will need to be changed by - 0.25 diopters. For each +0.50 diopter of cylindrical power removed, the spherical power will be changed by +0.25 diopters.
7. Recheck the sphere by presenting + 0.25 sphere and - 0.25 sphere.
8. Determine the best-corrected visual acuity on the appropriate ETDRS chart from the phoropter.
Personnel and Training Required
Trained ophthalmic technician or ophthalmologist
Equipment Needs
Humphrey Automatic Refractor (Carl Zeiss Meditec, Dublin, CA)
Note: This protocol uses the Humphrey Automated Refractor (Carl Zeiss Meditec, Dublin, CA). Other instruments can be used to measure refractive error. If other instruments are used, the reproducibility of the measurements should be comparable to those acquired with this protocol. In addition, when other instruments are used to collect these measurements, the manufacturer and model of equipment should be recorded. These other devices may require some different steps than are described in this protocol. Investigators should follow the equipment manufacturers instructions to ensure quality control.
Requirements
| Requirement Category | Required |
|---|---|
| Major equipment | Yes |
| Specialized training | Yes |
| Specialized requirements for biospecimen collection | No |
| Average time of greater than 15 minutes in an unaffected individual | No |
Mode of Administration
Clinical Measurement
Lifestage
Adult
Participants
Adults aged ≥ 40 years*
* While this protocol was used in a study of adults aged ≥40 years, the Ocular Working Group suggests that the same methodology can be used for individuals aged 18 years and older.
Selection Rationale
This assessment will provide information on rates and types of refractive errors found in adults. Refractive errors, especially significant levels can be genetically influenced. The protocol selected is a standard, well-characterized procedure especially designed for assessment in the adult population.
Language
Chinese, English
Standards
| Standard | Name | ID | Source |
|---|---|---|---|
| Logical Observation Identifiers Names and Codes (LOINC) | Refractive error adult proto | 62706-7 | LOINC |
| Human Phenotype Ontology | Abnormality of refraction | HP:0000539 | HPO |
| caDSR Form | PhenX PX111401 - Refractive Error Adult | 5973863 | caDSR Form |
Derived Variables
None
Process and Review
Not applicable.
Protocol Name from Source
University of Southern California, Los Angeles Latino Eye Study (LALES), 2000-2003
Source
University of Southern California, Los Angeles Latino Eye Study (LALES). 2000-2003.General References
Varma R, Paz SH, Azen SP, Klein R, Globe D, Torres M, Shufelt C, Preston-Martin S; Los Angeles Latino Eye Study Group. (2004). The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology, 111(6):1121-31.
Shufelt C, Fraser-Bell S, Ying-Lai M, Torres M, Varma R; Los Angeles Latino Eye Study Group. (2005). Refractive error, ocular biometry, and lens opalescence in an adult population: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci, 46(12):4450-60.
Protocol ID
111401
Variables
Export Variables| Variable Name | Variable ID | Variable Description | dbGaP Mapping | |
|---|---|---|---|---|
| PX111401_LeftEye_Best_Corrected_Visual_Acuity | ||||
| PX111401090000 | Left Eye Best Corrected Visual Acuity measured | N/A | ||
| PX111401_Left_Eye_Cylinder_Axis | ||||
| PX111401070000 | Left Eye Cylinder Axis measured | Variable Mapping | ||
| PX111401_Left_Eye_Cylindrical_Power | ||||
| PX111401080000 | Left Eye Cylindrical Power measured | Variable Mapping | ||
| PX111401_Left_Eye_Spherical_Power | ||||
| PX111401060000 | Left Eye Spherical Power measured | Variable Mapping | ||
| PX111401_Refractive_Error_Instrument_Model | ||||
| PX111401010000 | Model of instrument used to measure more | N/A | ||
| PX111401_RightEye_Best_Corrected_Visual_Acuity | ||||
| PX111401050000 | Right Eye Best Corrected Visual Acuity measured | N/A | ||
| PX111401_Right_Eye_Cylinder_Axis | ||||
| PX111401030000 | Right Eye Cylinder Axis measured | Variable Mapping | ||
| PX111401_Right_Eye_Cylindrical_Power | ||||
| PX111401040000 | Right Eye Cylindrical Power measured | Variable Mapping | ||
| PX111401_Right_Eye_Spherical_Power | ||||
| PX111401020000 | Right Eye Spherical Power measured | Variable Mapping | ||
Measure Name
Refractive Error Measurement
Release Date
February 26, 2010
Definition
Measurement of image focusing error of the eye (refractive error)
Purpose
Refractive error assessment is a critical component of a subject's ocular condition and health. It is a standard measurement in a routine ophthalmic evaluation. Refractive errors may be influenced by genetics.
Keywords
Ocular, Eye, Refractive error, Los Angeles Latino Eye Study, LALES, Multi-Ethnic Pediatric Eye Disease Study, MEPEDS, Baltimore Pediatric Eye Disease Study, BPEDS
Measure Protocols
| Protocol ID | Protocol Name |
|---|---|
| 111401 | Refractive Error - Adult |
| 111402 | Refractive Error - Children |
Publications
There are no publications listed for this protocol.