Protocol - Intraocular Pressure
Description
Intraocular pressure (IOP) is measured using the TONO-PEN® XL instrument. The cornea is prepared by introducing one drop of anesthetic onto the surface of the eye and the TONO-PEN® is used to obtain four valid IOP measurements. The instrument calculates an average and the examiner records the average. The instrument also provides the reliability level of the measurement. Only those readings that have a 5% variability level are recorded as they are considered to be accurate.
Specific Instructions
This protocol uses TONO-PEN® to measure intraocular pressure. This instrument has the advantages of portability and is relatively easy to use. Other hand-held instruments can be used to measure intraocular pressure. If other instruments are used, the reproducibility of the measurements should be comparable to those acquired with this protocol. In addition, when other instruments are used to collect these measurements, the manufacturer and model of equipment should be recorded. These other devices may require some different steps than are described in this protocol. Investigators should follow the equipment manufacturers instructions to ensure quality control.
Availability
This protocol is freely available; permission not required for use.
Protocol
Measuring Intraocular Pressure Using the TONO-PEN? XL.
Patient Preparation
The Ocu-Film located on the tip of the TONO-PEN? XL contains natural rubber LATEX which may cause allergic reactions. Question patients about allergies to Latex before examining them with the TONO-PEN? XL.
To prepare a patient for an IOP measurement:
1. Instill a drop of topical anesthetic onto the eye to be examined.
2. Position the patient, seated or supine, in front of a fixation target; or have the patient fixate on a point of reference (i.e. ear, nose, distant object) to minimize eye movement.
Note: The TONO-PEN? XL will function in any stable position.
Patient Examination
To Perform an IOP measurement:
1. Instruct the patient to look straight ahead at the fixation target with his/her eyes fully open.
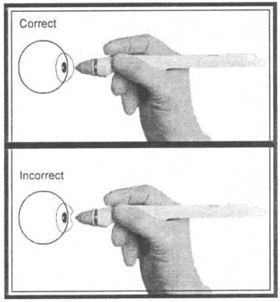
2. Hold the TONO-PEN? XL unit as you would a pencil.
3. Position yourself to facilitate viewing of the probe tip and patients cornea where contact will be made. For normal corneas, central corneal contact is recommended.
4. Brace the heel of your hand on the patients cheek for stability while holding the TONO-PEN? XL unit perpendicular to and within ? inch of the patients cornea.
5. To initiate an IOP measurement, depress the Operators Button once, and only once.
- Initially you will see a brief display of 18.8.8.81. This is a self-test of the LCD (Liquid Crystal Display). If any of the LCD segments are not displayed, the TONO-PEN? requires service.
- If a momentary display of ICALI is seen, followed immediately by a single row of dashes [- - - ], it indicated that the TONO-PEN? requires calibration before it will measure.
- If a double row of dashes is seen and a "beep" tone is heard, it indicates that the TONO-PEN? is ready to measure IOP. Proceed with applanation within 15 seconds.
6. Once active, after [ ==== ] is displayed and a "beep" tone is heard, touch the TONO-PEN? XL unit to the cornea lightly and briefly, then withdraw. Repeat several times. The corneal surface needs only to be momentarily contacted: indentation is not required and may lead to inaccurate readings.
7. A chirp will sound and a digital IOP measurement will be displayed each time a valid reading is obtained. The single horizontal bar at the bottom of the LCD, indicating statistical reliability, will not be displayed with each single IOP measurement.
8. After four (4) valid readings are obtained, a final beep will sound and the averaged measurement will appear on the LCD along with single bar denoting statistical reliability.
9. To take another measurement, reactivate the TONO-PEN? XL unit by pressing and releasing the activation switch as described in step 5.
10. Replace the Ocu-Film Tip Cover before using the TONO-PEN? XL unit on another patient and before storage.
Criteria for Emergency Referral
Any participant with intraocular pressure greater than 40 mm Hg should be referred immediately to a medical center. Notify the medical center by phone that the participant is on the way and that the patient has a high intraocular pressure and may need a laser iridotomy.
Criteria for Non-emergent Referral
Any participant with intraocular pressures between 21 mm Hg and 40 mm Hg or a potentially occludable angle should be referred to an ophthalmologist for a glaucoma-suspect work-up. The participant will be educated as to the seriousness of further testing and follow-up.
Personnel and Training Required
Trained ophthalmic technicians or ophthalmologists
Equipment Needs
TONO-PEN® XL Applanation Tonometer (Reichert Ophthalmic Instruments, Depew, NY)
TONO-PEN® is a registered trademark Reichert, Inc.
Note: This protocol uses TONO-PEN® to measure intraocular pressure. This instrument has the advantages of portability and is relatively easy to use. Other hand-held instruments can be used to measure intraocular pressure. If other instruments are used, the reproducibility of the measurements should be comparable to those acquired with this protocol. In addition, when other instruments are used to collect these measurements, the manufacturer and model of equipment should be recorded. These other devices may require some different steps than are described in this protocol. Investigators should follow the equipment manufacturers instructions to ensure quality control.
Requirements
| Requirement Category | Required |
|---|---|
| Major equipment | Yes |
| Specialized training | Yes |
| Specialized requirements for biospecimen collection | No |
| Average time of greater than 15 minutes in an unaffected individual | No |
Mode of Administration
Clinical Measurement
Lifestage
Child, Adolescent, Adult
Participants
Adults aged ≥ 40 years*
*While this protocol was used in a study of adults aged ≥ 40 years, the Ocular Working Group suggests that the same methodology can be used for individuals below age 40 and for children who are old enough to be able to tolerate the exam (usually by age 5). The WG notes that this methodology can also be used in children younger than 5 years, but that general anesthesia may be necessary.
Selection Rationale
The TONO-PEN® is an easy to use, portable hand-held electronic, digital pen-like instrument that determines IOP by making contact with the cornea, after use of topical anesthetic eye drops. This is especially useful for very young children and patients unable to reach a slit lamp due to disability. Measurement of intraocular pressure using this instrument does not require an office setting. It provides readings that correlate closely with Goldmann Tonometry, which was considered by the PhenX Working Group but was deemed to be of too high of burden for use by investigators who are not ocular experts.
Language
Chinese, English
Standards
| Standard | Name | ID | Source |
|---|---|---|---|
| Logical Observation Identifiers Names and Codes (LOINC) | Intraocular pressure proto | 62691-1 | LOINC |
| Human Phenotype Ontology | Abnormal intraocular pressure | HP:0012632 | HPO |
| caDSR Form | PhenX PX110701 - Intraocular Pressure | 5972137 | caDSR Form |
Derived Variables
None
Process and Review
Not applicable.
Protocol Name from Source
University of Southern California, The Los Angeles Latino Eye Study (LALES), 2000-2003
Source
University of Southern California, The Los Angeles Latino Eye Study (LALES). 2000-2003.General References
Varma R, Paz SH, Azen SP, Klein R, Globe D, Torres M, Shufelt C, Preston-Martin S; Los Angeles Latino Eye Study Group. (2004). The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology, 111(6):1121-31.
Protocol ID
110701
Variables
Export Variables| Variable Name | Variable ID | Variable Description | dbGaP Mapping | |
|---|---|---|---|---|
| PX110701_Intraocular_Pressure_Tono_Pen_Model | ||||
| PX110701010000 | Intraocular Pressure Tono-Pen Model | N/A | ||
| PX110701_Left_Eye_Intraocular_Pressure_1 | ||||
| PX110701040100 | Left Eye Intraocular Pressure measured, more | N/A | ||
| PX110701_Left_Eye_Intraocular_Pressure_2 | ||||
| PX110701040200 | Left Eye Intraocular Pressure measured, more | N/A | ||
| PX110701_Left_Eye_Intraocular_Pressure_3 | ||||
| PX110701040300 | Left Eye Intraocular Pressure measured, more | N/A | ||
| PX110701_Left_Eye_Intraocular_Pressure_4 | ||||
| PX110701040400 | Left Eye Intraocular Pressure measured, more | N/A | ||
| PX110701_Left_Eye_Statistical_Reliability_Level1 | ||||
| PX110701050100 | Left Eye Statistical Reliability Level more | N/A | ||
| PX110701_Left_Eye_Statistical_Reliability_Level2 | ||||
| PX110701050200 | Left Eye Statistical Reliability Level more | N/A | ||
| PX110701_Left_Eye_Statistical_Reliability_Level3 | ||||
| PX110701050300 | Left Eye Statistical Reliability Level more | N/A | ||
| PX110701_Left_Eye_Statistical_Reliability_Level4 | ||||
| PX110701050400 | Left Eye Statistical Reliability Level more | N/A | ||
| PX110701_Right_Eye_Intraocular_Pressure_1 | ||||
| PX110701020100 | Right Eye Intraocular Pressure measured, more | Variable Mapping | ||
| PX110701_Right_Eye_Intraocular_Pressure_2 | ||||
| PX110701020200 | Right Eye Intraocular Pressure measured, more | N/A | ||
| PX110701_Right_Eye_Intraocular_Pressure_3 | ||||
| PX110701020300 | Right Eye Intraocular Pressure measured, more | N/A | ||
| PX110701_Right_Eye_Intraocular_Pressure_4 | ||||
| PX110701020400 | Right Eye Intraocular Pressure measured, more | N/A | ||
| PX110701_Right_Eye_Statistical_Reliability_Level1 | ||||
| PX110701030100 | Right Eye Statistical Reliability Level more | N/A | ||
| PX110701_Right_Eye_Statistical_Reliability_Level2 | ||||
| PX110701030200 | Right Eye Statistical Reliability Level more | N/A | ||
| PX110701_Right_Eye_Statistical_Reliability_Level3 | ||||
| PX110701030300 | Right Eye Statistical Reliability Level more | N/A | ||
| PX110701_Right_Eye_Statistical_Reliability_Level4 | ||||
| PX110701030400 | Right Eye Statistical Reliability Level more | N/A | ||
Measure Name
Intraocular Pressure
Release Date
February 26, 2010
Definition
Procedure for the measurement of intraocular pressure (IOP) using the TONO-PEN® XL Applanation Tonometer.
TONO-PEN® is a registered trademark Reichert, Inc.
Purpose
This procedure is used to measure intraocular pressure (IOP). An elevated IOP can be dangerous because people with varying degrees of IOP elevation may develop damage to the optic nerve.
Keywords
Ocular, Eye, Intraocular pressure, IOP, Los Angeles Latino Eye Study, LALES
Measure Protocols
| Protocol ID | Protocol Name |
|---|---|
| 110701 | Intraocular Pressure |