Protocol - Myocardial Infarction
- Blood Pressure (Adult/Primary)
- Cigarette Smoking Status - Adolescent
- Cigarette Smoking Status - Adult
- Lipid Profile
- Weight - Measured Weight
- Weight - Self-Reported Weight
Description
This measure assesses if an individual has had a myocardial infarction (MI) through collection of personal history of disease, treatment and procedure histories, and medical record abstraction.
Specific Instructions
If the respondent answers "yes" to question 1 or question 2, the interviewer should complete the rest of the protocol. If the respondent answers "no" or "dont know," then the protocol is deemed complete.
Availability
This protocol is freely available; permission not required for use.
Protocol
1. Has a doctor ever told you that you had a myocardial infarction or heart attack?
[ ] 1 Yes
[ ] 0 No
[ ] 9 Dont Know
2. Have you had an outpatient or day surgery procedure to unblock blocked or narrowed blood vessels of the heart (called a PTCA, coronary angioplasty, stent, or atherectomy)?
[ ] 1 Yes
[ ] 0 No
Remainder of protocol to be abstracted from patients hospital medical records.
3. Was there an acute episode of pain, discomfort or tightness in the chest, left arm or jaw within 72 hours of the hospitalization or within 72 hours of the in-hospital event?
[ ] Yes
[ ] No
[ ] Unknown
4. Was the discomfort or pain diagnosed as having a non-cardiac origin?
[ ] Yes
[ ] No
[ ] Unknown
5. Were electrocardiograms (ECGs or EKGs) recorded?
[ ] Yes
[ ] No
[ ] Unknown
If "No" or "Unknown," skip to 6.
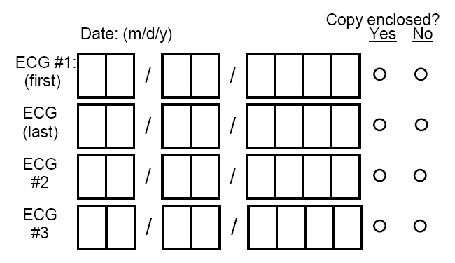
Record dates of ECGs and make two copies of FOUR ECG tracings as described below. Send one copy to the ECG Reading Center and attach one copy to this form:
-- If four or fewer tracings were made, include all tracings.
-- If more than four tracings were made, include:
1. First two codable tracings after admission (ECG#1-First and ECG#2)
2. Last codable tracing prior to discharge or death (discharge tracing) (ECG-Last)
3. Last codable tracing on day 3 (or the first tracing thereafter) following an admission or in-hospital event (ECG#3)
4. The next codable tracing after day 3
-- If the participant is readmitted (transferred) to the ICU/CCU because of a new episode of chest pain, the first codable tracing may be sent.
Serum Enzymes
6. Were any cardiac enzyme measurements performed during this admission?
[ ] Yes
[ ] No
If "No," skip to end.
7. Did the participant have any active liver disease (cirrhosis, hepatitis, liver cancer, etc.)?
[ ] Yes
[ ] No
If "Yes," specify:
_____________________________________________________
8. Is there any evidence of hemolytic disease during this hospitalization?
[ ] Yes
[ ] No
9. Is there any mention of the participant having either trauma, a _ procedure, or rhabdomyolysis within one week prior to the measurement of the cardiac enzymes?
[ ] Yes
[ ] No
[ ] Unknown
If "Yes," please specify type of trauma or procedure below.
Date m/d/y: __ / __ / __ __
Type of Trauma or procedure:
__________________________________________
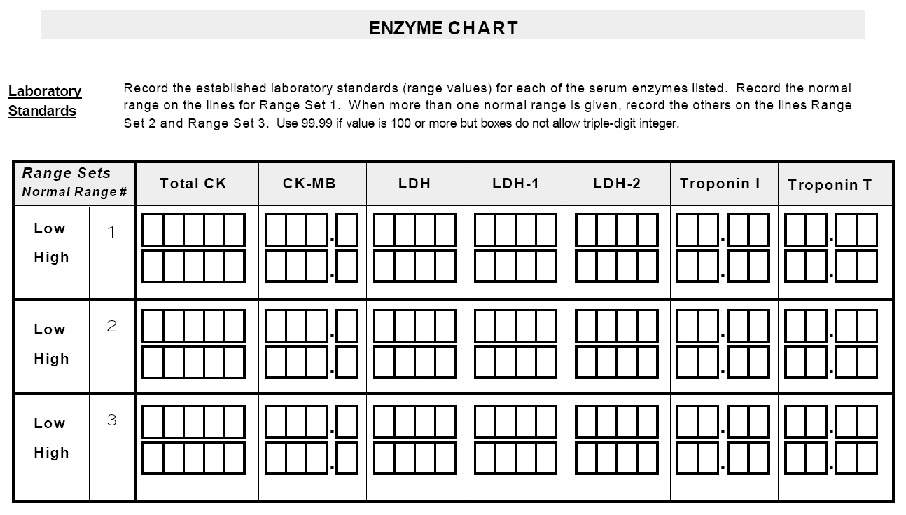
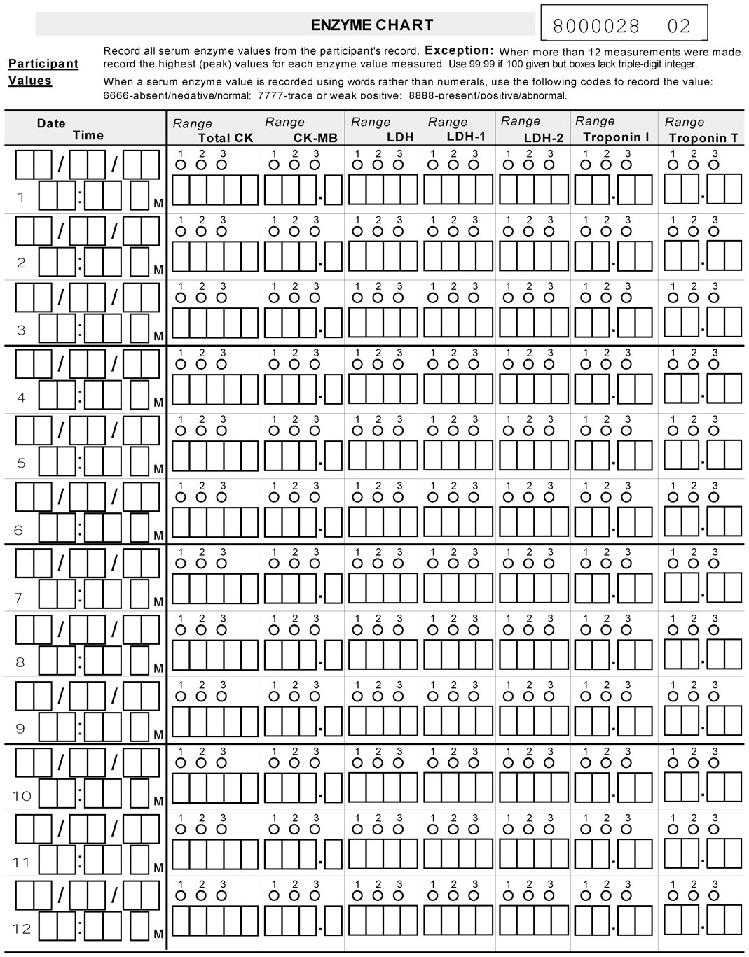
* Please complete ENZYME CHART. *
10. Myocardial infarction
[ ] Definite
[ ] Probable
[ ] No MI (skip to end)
If “Definite” or “Probable” enter date of MI (MM/DD/YYYY): __ __ / __ __ / __ __ __ __
Criteria
11. Chest Pain
[ ] Present
[ ] Absent
12. Cardiac Enzymes
[ ] Abnormal
[ ] Equivocal
[ ] Incomplete
[ ] Normal
13. ECG Serial Reading (pick one)
[ ] Evolution of Major Q-Wave
[ ] Evolution of ST-T Elevation with or without Q-Wave
[ ] New LBBB
[ ] Evolution of ST-Depression/Inversion alone
[ ] Evolution of Minor Q-Wave alone
[ ] Single ECG with Major Q-Wave
[ ] Single ECG with LBBB, described as new
[ ] Absent, Uncodable or Other ECG
14. Procedure-related:
[ ] Yes, cardiovascular
[ ] Yes, non-cardiovascular
[ ] No
Diagnostic Criteria:
Acute, Evolving or Recent MI
Either one of the following criteria satisfies the diagnosis for an acute, evolving or recent MI:
1. Typical rise and gradual fall (troponin) or more rapid rise and fall (CK-MB) of biochemical markers of myocardial necrosis with at least one of the following:
- ischemic symptoms;
- development of pathologic Q waves on the ECG;
- ECG changes indicative of ischemia (ST segment elevation or depression); or
- coronary artery intervention (e.g., coronary angioplasty).
2. Pathologic findings of an acute MI.
Established MI
Any one of the following criteria satisfies the diagnosis for established MI:
1. Development of new pathologic Q waves on serial ECGs. The patient may or may not remember previous symptoms. Biochemical markers of myocardial necrosis may have normalized, depending on the length of time that has passed since the infarct developed.
2. Pathologic findings of a healed or healing MI.
Personnel and Training Required
An interviewer who is trained to conduct personal interviews with individuals from the general population is required to complete the protocols from the Cardiovascular Health Study (CHS) and the Womens Health Initiative (WHI). The interviewer must be trained and found to be competent (i.e., tested by an expert) at the completion of personal interviews.* The interviewer should be trained to prompt respondents further if a "dont know" response is provided.
*There are multiple modes to administer this question (e.g., paper-and-pencil and computer-assisted interviews).
A trained and certified Hospital Record Abstractor is required to complete the protocol from the Multi-Ethnic Study of Atherosclerosis (MESA). This person should be trained to perform medical record and chart abstraction using various hospital records.
Equipment Needs
None
Requirements
| Requirement Category | Required |
|---|---|
| Major equipment | No |
| Specialized training | Yes |
| Specialized requirements for biospecimen collection | No |
| Average time of greater than 15 minutes in an unaffected individual | No |
Mode of Administration
Interviewer-administered question, Medical record abstraction
Lifestage
Adult
Participants
Cardiovascular Health Study (CHS): >65 years old
Womens Health Initiative (WHI): women ages 50–79 years old*
Multi-Ethnic Study of Atherosclerosis (MESA): ages 45–85 years old
*While this questionnaire was used in a womens study, the Cardiovascular Working Group deems it appropriate to use with men.
Selection Rationale
A combination of four protocols was chosen to obtain sufficient data to assess whether a person had a myocardial infarction. Obtaining a personal history of myocardial infarction and treatment procedures, in combination with medical record abstraction, will provide a high level of specificity and reliability.
Language
Chinese, English, Other languages available at source
Standards
| Standard | Name | ID | Source |
|---|---|---|---|
| Logical Observation Identifiers Names and Codes (LOINC) | Myocardial infarct proto | 62397-5 | LOINC |
| Human Phenotype Ontology | Myocardial infarction | HP:0001658 | HPO |
| caDSR Form | PhenX PX040801 - Myocardial Infarction | 5838015 | caDSR Form |
Derived Variables
None
Process and Review
Not applicable.
Protocol Name from Source
Cardiovascular Health Study (CHS), Baseline Medical History Questionnaire & Womens Health Initiative (WHI), Medical History Update
Source
U.S. Department of Health and Human Services. National Institutes of Health. National Heart, Lung and Blood Institute. Cardiovascular haHealth Study (CHS). Baseline Medical History Questionnaire. Page 1. Question 1 (source for question 1).
U.S. Department of Health and Human Services. National Institutes of Health. National Heart, Lung and Blood Institute. Womens Health Initiative (WHI). Form 33D—Medical History Update. Version 4. Question 3.3 (source for question 2).
U.S. Department of Health and Human Services. National Institutes of Health. National Heart, Lung and Blood Institute. Multi-Ethnic Study of Atherosclerosis (MESA). Hospital Abstraction: Cardiac/Peripheral Vascular Disease (PVD) Form. Questions 15, 17, 27–31 (source for questions 3–9).
U.S. Department of Health and Human Services. National Institutes of Health. National Heart, Lung and Blood Institute. Multi-Ethnic Study of Atherosclerosis (MESA). Cardiac Review Form. Question 1 (source for questions 10–14 ).
Alpert, J. S., Thygesen, K., Antman, E., & Bassand, J. P. (2000). Myocardial infarction redefined—A consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Journal of the American College of Cardiology, 36(3), 959–969 (source for Diagnostic Criteria).
General References
Ives, D. G., Fitzpatrick, A. L., Bild, D. E., Psaty, B. M., Kuller, L. H., Crowley, P. M., Cruise, R. G., & Theroux, S. (1995). Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Annals of Epidemiology, 5, 278–285.
Psaty, B. M., Kuller, L. H., Bild, D., Burke, G. L., Kittner, S. J., Mittelmark, M., Price, T. R., Rautaharju, P. M., & Robbins, J. (1995). Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Annals of Epidemiology, 5(4), 270–277.
Protocol ID
40801
Variables
Export Variables| Variable Name | Variable ID | Variable Description | dbGaP Mapping | |
|---|---|---|---|---|
| PX040801_Cardiac_Enzyme | ||||
| PX040801120000 | Cardiac Enzymes | N/A | ||
| PX040801_Chest_Discomfort | ||||
| PX040801030000 | Was there an acute episode of pain, more
discomfort or tightness in the chest, left arm or jaw within 72 hours of the hospitalization or within 72 hours of the in-hospital event? show less
|
Variable Mapping | ||
| PX040801_Chest_Discomfort_Non_Cardiac | ||||
| PX040801040000 | Was the discomfort or pain diagnosed as more
having a non-cardiac origin? show less
|
Variable Mapping | ||
| PX040801_Chest_Pain | ||||
| PX040801110000 | Chest Pain | Variable Mapping | ||
| PX040801_ECG_Serial_Reading | ||||
| PX040801130000 | ECG Serial Reading (pick one) | N/A | ||
| PX040801_Electrocardiogram | ||||
| PX040801050000 | Were electrocardiograms (ECGs or EKGs) recorded? | Variable Mapping | ||
| PX040801_Enzyme_Chart_Date | ||||
| PX040801090700 | Record all serum enzyme values Date (mm/dd/yy) | N/A | ||
| PX040801_Enzyme_Chart_Enzyme | ||||
| PX040801090300 | Record all serum enzyme values Serum enzyme | N/A | ||
| PX040801_Enzyme_Chart_Range | ||||
| PX040801090400 | Record all serum enzyme values Normal Range | N/A | ||
| PX040801_Enzyme_Chart_Time | ||||
| PX040801090800 | Record all serum enzyme values Time (hh:mm am/pm) | N/A | ||
| PX040801_Enzyme_Chart_Value_High | ||||
| PX040801090600 | Record all serum enzyme values Highest more
enzyme value show less
|
N/A | ||
| PX040801_Enzyme_Chart_Value_Low | ||||
| PX040801090500 | Record all serum enzyme values Lowest enzyme value | N/A | ||
| PX040801_Ever_Have_Myocardial_Infarction | ||||
| PX040801010000 | Has a doctor ever told you that you had a more
myocardial infarction or heart attack? show less
|
Variable Mapping | ||
| PX040801_Ever_Have_Stent | ||||
| PX040801020000 | Have you had an outpatient or day surgery more
procedure to unblock blocked or narrowed blood vessels of the heart (called a PTCA, coronary angioplasty, stent, or atherectomy)? show less
|
Variable Mapping | ||
| PX040801_Evidence_Hemolytic_Disease | ||||
| PX040801080000 | Is there any evidence of hemolytic disease more
during this hospitalization? show less
|
N/A | ||
| PX040801_Liver_Disease | ||||
| PX040801070000 | Did the participant have any active liver more
disease (cirrhosis, hepatitis, liver cancer, etc.)? show less
|
Variable Mapping | ||
| PX040801_Liver_Disease_Specify | ||||
| PX040801070100 | Did the participant have any active liver more
disease (cirrhosis, hepatitis, liver cancer, etc.)? If Yes, specify. show less
|
Variable Mapping | ||
| PX040801_Myocardial_Infarction | ||||
| PX040801100000 | Myocardial infarction | Variable Mapping | ||
| PX040801_Myocardial_Infarction_Date | ||||
| PX040801100100 | Myocardial infarction: If "Definite" or more
"Probable" enter date of MI. show less
|
Variable Mapping | ||
| PX040801_Procedure_Related | ||||
| PX040801140000 | Procedure-related | N/A | ||
| PX040801_Serum_Enzyme | ||||
| PX040801060000 | Were any cardiac enzyme measurements more
performed during this admission? show less
|
N/A | ||
| PX040801_Trauma_Before_Enzyme_Measurement | ||||
| PX040801090000 | Is there any mention of the participant more
having either trauma, a surgical procedure, or rhabdomyolysis within one week prior to the measurement of the cardiac enzymes? show less
|
N/A | ||
| PX040801_Trauma_Before_Enzyme_Measurement_Date | ||||
| PX040801090100 | Is there any mention of the participant more
having either trauma, a surgical procedure, or rhabdomyolysis within one week prior to the measurement of the cardiac enzymes? If Yes, specify date of trauma or procedure. show less
|
N/A | ||
| PX040801_Trauma_Before_Enzyme_Measurement_Type | ||||
| PX040801090200 | Is there any mention of the participant more
having either trauma, a surgical procedure, or rhabdomyolysis within one week prior to the measurement of the cardiac enzymes? If Yes, specify type of trauma or procedure. show less
|
N/A | ||
Measure Name
Myocardial Infarction
Release Date
September 9, 2009
Definition
Measure to assess if patient had a myocardial infarction (heart attack).
Purpose
Myocardial infarction (heart attack) is a leading cause of death for both men and women in the United States, which makes myocardial infarction a prevalent health issue for investigators to study.
Keywords
Cardiovascular, myocardial infarction, Heart attack, Multi-Ethnic Study of Atherosclerosis, MESA, Cardiovascular Health Study, CHS, Womens Health Initiative, WHI, chest pain, percutaneous transluminal coronary angioplasty, PTCA, coronary angioplasty, atherectomy, hospitalization, coronary stent, thrombolytic therapy, electrocardiogram, ECG, EKG, myocardial ischemia, ST-elevation myocardial infarction, STEMI, anticoagulation, coronary bypass surgery, personal history
Measure Protocols
| Protocol ID | Protocol Name |
|---|---|
| 40801 | Myocardial Infarction |
Mapped dbGaP Studies
Mapping for PX040801_Chest_Discomfort
| dbGAP Study | dbGAP Variable | dbGAP Variable Description |
|---|---|---|
| The Genomics and Randomized Trials Network (GARNET) Vitamin Intervention Stroke Prevention (VISP) trial | phv00179919 | Was there an acute episode(s) of discomfort or pain anywhere in the chest, left arm or jaw, within 72 hours prior to arrival or in conjunction with the in-hospital CHD event? |
Mapped dbGaP Studies
Mapping for PX040801_Chest_Discomfort_Non_Cardiac
| dbGAP Study | dbGAP Variable | dbGAP Variable Description |
|---|---|---|
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00203781 | Was the discomfort or pain diagnosed as having a non-cardiac origin? Q11d [Coroner / Medical Examiner Form] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00203918 | [Second transfer]. Was the discomfort or pain diagnosed as having a non-cardiac origin? Q25d [Hospital Abstraction Form] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00203926 | [Second transfer]. Non-cardiac source of chest pain. Q25e [Hospital Abstraction Form] |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00048764 | J2b2. Verification of event, chest pain: definite non-cardiac cause for pain |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00049182 | J2b2. Verification of event, chest pain: definite non-cardiac cause for pain |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00051016 | C2b. Non-cardiac cause for the pain |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00051057 | C2b. Non-cardiac cause for the pain |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00066214 | C2. Non-cardiac cause for the pain |
| The Genomics and Randomized Trials Network (GARNET) Vitamin Intervention Stroke Prevention (VISP) trial | phv00168538 | Was the discomfort or pain diagnosed as having a non-cardiac origin? |
| The Genomics and Randomized Trials Network (GARNET) Vitamin Intervention Stroke Prevention (VISP) trial | phv00179923 | Was the discomfort or pain diagnosed as having a non-cardiac origin? |
Mapped dbGaP Studies
Mapping for PX040801_Chest_Pain
| dbGAP Study | dbGAP Variable | dbGAP Variable Description |
|---|---|---|
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00202877 | [Chest pain on effort]. Have you had any pain or discomfort in your chest? [Annual Follow-up Questionnaire Form, AFUA] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00207761 | [Chest pain on effort]. Doctor diagnosis of chest pain. Q13 [Medical History Form, exam 1] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00207775 | [Possible infraction]. Doctor diagnosis of Q25 chest pain. Q27 [Medical History Form, exam 1] |
| Cardiovascular Health Study (CHS) Cohort | phv00099137 | PRESENT CHEST PAIN |
| Cardiovascular Health Study (CHS) Cohort | phv00110421 | EXPERIENCING CHEST PAIN |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00029184 | Vascular complications: chest pain |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00066203 | C. Chest pain. If no pain skip |
| Jackson Heart Study (JHS) Cohort | phv00124742 | 1a. Chest pain or angina |
| Jackson Heart Study (JHS) Cohort | phv00124866 | 1a. Chest pain or angina |
| NHLBI Cleveland Family Study (CFS) Candidate Gene Association Resource (CARe) | phv00123464 | 86 Ever had any chest pain or discomfort |
| OPPERA baseline case-control study of chronic TMJD | phv00223223 | 15. Chest Pains |
| OPPERA Prospective Cohort Study of First-Onset TMJD | phv00224294 | 15. Chest Pains |
Mapped dbGaP Studies
Mapping for PX040801_Electrocardiogram
| dbGAP Study | dbGAP Variable | dbGAP Variable Description |
|---|---|---|
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00205675 | ECGMB Record Present [Cohort, Exam 2] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00205730 | ECGMC record present [ECG Composite 12 Lead (with adjudications as needed) (ECGMC35), exam 3] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00205787 | ECGMD record present [ECG Composite 12 Lead (with adjudications as needed) (ECGMD41), exam 4] |
Mapped dbGaP Studies
Mapping for PX040801_Ever_Have_Myocardial_Infarction
| dbGAP Study | dbGAP Variable | dbGAP Variable Description |
|---|---|---|
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00094310 | HEART ATTACK-DIAGNOSED BY DOCTOR Q5C |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00092946 | V4 MD Diagnosed Myocardial Infarction |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00092853 | V3 MD Diagnosed Myocardial Infarction |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00092772 | V2 MD Diagnosed Myocardial Infarction |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00092711 | MD DIAGNOSED MYOCARDIAL INFARCTION |
| CARDIA Cohort | phv00116960 | HEART ATTACK? Q 3 |
| CARDIA Cohort | phv00114389 | HAD HEART ATTACK? Q 1 |
| CARDIA Cohort | phv00113025 | HAS/HAD HEART ATTACK? |
| Cardiovascular Health Study (CHS) Cohort | phv00197449 | MD said pt had heart attack? |
| Cardiovascular Health Study (CHS) Cohort | phv00099015 | MYOCARDIAL INFARCTION OR HEART ATTACK |
| Cardiovascular Health Study (CHS) Cohort | phv00098772 | Family History of heart attack |
| Framingham Cohort | phv00021036 | HAVE YOU EVER BEEN TOLD BY A DOCTOR YOU HAD A HEART ATTACK OR MYOCARDIAL INFARCTION? |
| GenADA/LONG/Imaging (Genetic Alzheimer's Disease Associations) | phv00081690 | HEART ATTACK, ANGINA: Family history of disease |
| GenADA/LONG/Imaging (Genetic Alzheimer's Disease Associations) | phv00081689 | HEART ATTACK, ANGINA: Subject history of disease |
| GenADA/LONG/Imaging (Genetic Alzheimer's Disease Associations) | phv00081546 | HEART ATTACK, ANGINA: Family History of disease |
| GenADA/LONG/Imaging (Genetic Alzheimer's Disease Associations) | phv00081545 | HEART ATTACK, ANGINA: Subject History of disease |
| GenADA/LONG/Imaging (Genetic Alzheimer's Disease Associations) | phv00081424 | HEART ATTACK, ANGINA: Family History of disease |
| GenADA/LONG/Imaging (Genetic Alzheimer's Disease Associations) | phv00081423 | HEART ATTACK, ANGINA: Subject History of disease |
| Genetic Epidemiology of COPD (COPDGene) | phv00159615 | Heart attack [MI] |
| Genetic Multiple Sclerosis Associations - GeneMSA | phv00073060 | Subject's history of disease - HEART ATTACK - ANGINA |
| Genetic Multiple Sclerosis Associations - GeneMSA | phv00073061 | Family history of disease - HEART ATTACK - ANGINA |
| GENEVA Genetics of Early Onset Stroke (GEOS) Study | phv00111934 | Has a doctor ever told you that you have myocardial infarction or heart attack? |
| Genome Wide Association Study of Chronic TMD: Discovery Phase | phv00260445 | MED 1. Cardiovascular conditions: heart attack. |
| Genome-wide Association Study of Adiposity in Samoans | phv00258767 | Have you ever had a heart attack? Self-report |
| Hispanic Community Health Study /Study of Latinos (HCHS/SOL) | phv00258068 | Heart attack - self (MHEA4) |
| Jackson Heart Study (JHS) Cohort | phv00124993 | 7a. Heart attack |
| Jackson Heart Study (JHS) Cohort | phv00128091 | Q4a. Has doctor said you had heart attack? [Visit 1] [Personal and Family Health History Form, PFH] |
| Jackson Heart Study (JHS) Cohort | phv00126085 | History of MD diagnosed MI |
| Jackson Heart Study (JHS) Cohort | phv00125773 | DOCTOR - HEART ATTACK Q7A |
| Jackson Heart Study (JHS) Cohort | phv00125685 | DOCTOR - HEART ATTACK Q7A |
| Jackson Heart Study (JHS) Cohort | phv00125597 | DOCTOR - HEART ATTACK Q7A |
| Jackson Heart Study (JHS) Cohort | phv00125509 | DOCTOR - HEART ATTACK Q7A |
| Jackson Heart Study (JHS) Cohort | phv00125249 | 7a. Heart attack |
| Jackson Heart Study (JHS) Cohort | phv00125163 | 7a. Heart attack |
| Jackson Heart Study (JHS) Cohort | phv00125078 | 7a. Heart attack |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00085321 | FAMILY HISTORY OF HEART ATTACK (PARENTS/SIBLINGS/CHILDREN) |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087142 | HEART ATTACK |
| NHGRI Genome-Wide Association Study of Venous Thromboembolism (GWAS of VTE) | phv00121850 | Ever had a heart attack (mi) |
| NHLBI Cleveland Family Study (CFS) Candidate Gene Association Resource (CARe) | phv00122274 | Heart attack diagnosed by doctor (A) |
| NHLBI Cleveland Family Study (CFS) Candidate Gene Association Resource (CARe) | phv00122273 | Heart attack (A) |
| NIA Long Life Family Study (LLFS) | phv00163268 | Myocardial Infarction or Heart Attack diagnosed? |
| The Genomics and Randomized Trials Network (GARNET) Vitamin Intervention Stroke Prevention (VISP) trial | phv00179610 | Has a doctor ever said you had any of the following? Heart attack |
| Whole Genome Association Study of Bipolar Disorder | phv00017032 | B1. HeartAttack, Yes/No. (Participants with European ancestry). DIGS4 |
| Whole Genome Association Study of Bipolar Disorder | phv00051936 | B1 Heart attack, Yes/No, (African American participants). DIGS4 |
| Women's Health Initiative | phv00078684 | Relatives had heart attack |
Mapped dbGaP Studies
Mapping for PX040801_Ever_Have_Stent
| dbGAP Study | dbGAP Variable | dbGAP Variable Description |
|---|---|---|
| CARDIA Cohort | phv00121382 | PTCA, CORONARY STENT OR ATHERECTOMY? |
| CARDIA Cohort | phv00121602 | PTCA, CORONARY STENT OR ATHERECTOMY? |
| Framingham Cohort | phv00072203 | CORONARY ARTERY ANGIOPLASTY/STENT/PCI |
| Framingham Cohort | phv00072206 | CAROTID ARTERY SURGERY/STENT |
| Framingham Cohort | phv00072208 | ABDOMINAL AORTA SURGERY/STENT |
| Framingham Cohort | phv00072209 | FEMORAL OR LOWER EXTREMITY SURGERY/STENT/ANGIOPLASTY |
| Jackson Heart Study (JHS) Cohort | phv00125463 | 45a. Angioplasty or stent of the coronary arteries |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087862 | PTCA, COR. STENT, OR COR. ATHERECTOMY |
Mapped dbGaP Studies
Mapping for PX040801_Liver_Disease
| dbGAP Study | dbGAP Variable | dbGAP Variable Description |
|---|---|---|
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00091329 | CIRRHOSIS OR LIVER DISEASE Q2E |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00206812 | Section 3: Medical History. Q12C - Gastro/Endo his: Liver disease [Heart Failure Hospital Record Abstraction Form, HFA] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00203765 | LIVER DISEASE? Q7G [Coroner /Medical Examiner Form] |
| CARDIA Cohort | phv00118460 | LIVER DISEASE? Q 6 |
| CARDIA Cohort | phv00113093 | HAS/HAD OTHER LIVER DISEASE? |
| CARDIA Cohort | phv00114443 | HAS(/D) OTHER LIVER DISEASE? Q 3 |
| CARDIA Cohort | phv00116986 | LIVER DISEASE. Q 6 |
| CARDIA Cohort | phv00119822 | LIVER DISEASE? Q 6 |
| Cardiovascular Health Study (CHS) Cohort | phv00104171 | LIVER DISEASE, CIRRHOSIS OR HEPATITIS |
| Cardiovascular Health Study (CHS) Cohort | phv00102722 | HAVE LIVER DISEASE, CIRRHOSIS, HEPATITIS |
| Cardiovascular Health Study (CHS) Cohort | phv00101545 | HAVE LIVER DISEASE, CIRRHOSIS, HEPATITIS |
| Cardiovascular Health Study (CHS) Cohort | phv00110066 | HAVE LIVER DISEASE, CIRRHOSIS, HEPATITIS |
| Cardiovascular Health Study (CHS) Cohort | phv00109404 | HAVE LIVER DISEASE, CIRRHOSIS, HEPATITIS |
| Cardiovascular Health Study (CHS) Cohort | phv00108666 | HAVE LIVER DISEASE, CIRRHOSIS, HEPATITIS |
| Cardiovascular Health Study (CHS) Cohort | phv00107992 | HAVE LIVER DISEASE, CIRRHOSIS, HEPATITIS |
| Cardiovascular Health Study (CHS) Cohort | phv00107097 | HAVE LIVER DISEASE, CIRRHOSIS, HEPATITIS |
| Cardiovascular Health Study (CHS) Cohort | phv00106079 | HAVE LIVER DISEASE, CIRRHOSIS, HEPATITIS |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00034365 | Patient has a history of: liver disease, jaundice |
| Framingham Cohort | phv00000527 | FINAL DIAGNOSTIC IMPRESSION: LIVER DISEASE, EXAM 1 |
| Framingham Cohort | phv00177524 | Clinical Diagnostic Impression: Liver disease |
| Framingham Cohort | phv00072321 | CLINICAL DIAGNOSTIC IMPRESSION: LIVER DISEASE |
| Framingham Cohort | phv00021213 | CDI - LIVER DISEASE |
| Genotype-Tissue Expression (GTEx) | phv00169171 | Liver Disease (liver abscess, failure, fatty liver syndrome, inherited liver insufficiency, acute/chronic hepatic insufficiency, necrobacillosis, rupture) |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087139 | LIVER DISEASE: HEPATITIS, TYPE UNKNOWN |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087137 | LIVER DISEASE: HEPATITIS, TYPE D |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00082987 | LIVER DISEASE: SELF-REPORT |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00085223 | LIVER DISEASE: SELF-REPORT |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00085939 | LIVER DISEASE: SELF-REPORT |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00086421 | LIVER DISEASE: SELF-REPORT |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087131 | LIVER DISEASE |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087133 | LIVER DISEASE: HEPATITIS |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087134 | LIVER DISEASE: HEPATITIS, TYPE A |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087135 | LIVER DISEASE: HEPATITIS, TYPE B |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087136 | LIVER DISEASE: HEPATITIS, TYPE C |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087138 | LIVER DISEASE: HEPATITIS, TYPE E |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087132 | LIVER DISEASE: CIRRHOSIS |
| NHLBI Cleveland Family Study (CFS) Candidate Gene Association Resource (CARe) | phv00123512 | 95 Liver Disease |
| NIDCR Sjogren's International Collaborative Clinical Alliance (SICCA) | phv00226913 | Physician confirmed Liver disease |
| NIDCR Sjogren's International Collaborative Clinical Alliance (SICCA) | phv00226908 | Self reported Liver disease |
| The Genomics and Randomized Trials Network (GARNET) Vitamin Intervention Stroke Prevention (VISP) trial | phv00168521 | Was the finding: Liver disease |
| Women's Health Initiative | phv00078183 | Liver disease |
| Women's Health Initiative | phv00078043 | Liver disease |
| Women's Health Initiative | phv00077879 | Liver disease |
| Women's Health Initiative | phv00077769 | Liver disease |
| Women's Health Initiative | phv00077644 | Liver disease |
| Women's Health Initiative | phv00078324 | Liver disease |
| Women's Health Initiative | phv00078487 | Liver disease ever |
Mapped dbGaP Studies
Mapping for PX040801_Liver_Disease_Specify
| dbGAP Study | dbGAP Variable | dbGAP Variable Description |
|---|---|---|
| CARDIA Cohort | phv00114444 | DESC OF OTHER LIVER DISEASE. Q 3 |
| CARDIA Cohort | phv00119980 | SPECIFY, OTHER LIVER DISEASE. Q 6 |
Mapped dbGaP Studies
Mapping for PX040801_Myocardial_Infarction
| dbGAP Study | dbGAP Variable | dbGAP Variable Description |
|---|---|---|
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00204935 | Visit 4, MD diagnosed myocardial infarction [Cohort, Exam 4] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00203786 | Is there a history of a myocardial infarction prior to the onset of this event? Q15a [Coroner / Medical Examiner Form] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00203907 | [Second transfer]. Is there mention of acute myocardial infarction [MI] in the discharge summary? Q20d [Hospital Abstraction Form] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00204613 | Visit 2, MD diagnosed myocardial infarction [Cohort, Exam 2] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00204614 | Visit 2, history of myocardial infarction [Cohort, Exam 2] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00204748 | MD diagnosed myocardial infarction [Cohort, Exam 1] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00204749 | History of myocardial infarction [Cohort, Exam 1] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00204831 | Visit 3, MD diagnosed myocardial infarction [Cohort, Exam 3] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00204832 | Visit 3, history of myocardial infarction [Cohort, Exam 3] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00204936 | Visit 4, history of myocardial infarction [Cohort, Exam 4] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00206546 | Did (name) ever have a heart attack? Q12 [Family History Form, exam 2] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00206554 | Did (name) ever have a heart attack? Q21 [Family History Form, exam 2] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00206562 | Did (name) ever have a heart attack? Q30 [Family History Form, exam 2] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00206570 | Did (name) ever have a heart attack? Q39 [Family History Form, exam 2] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00206578 | Did (name) ever have a heart attack? Q48 [Family History Form, exam 2] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00206805 | [Medical history]. Q11k - Myocardial infarction [Heart Failure Hospital Record Abstraction Form, HFA] |
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00207023 | [Medical history]. Q16o - Precipitating factor: myocardial infarction (removed from current form version) [Heart Failure Hospital Record Abstraction Form, HFA] |
| CARDIA Cohort | phv00113025 | HAS/HAD HEART ATTACK? |
| CARDIA Cohort | phv00114389 | HAD HEART ATTACK? Q 1 |
| CARDIA Cohort | phv00114391 | STATUS, HEART ATTACK. Q 1 |
| CARDIA Cohort | phv00115429 | HEART ATTACK. Q 3 |
| CARDIA Cohort | phv00116960 | HEART ATTACK? Q 3 |
| CARDIA Cohort | phv00117946 | HEART ATTACK? - MON 96 |
| CARDIA Cohort | phv00117973 | HEART ATTACK? - MON 108 |
| CARDIA Cohort | phv00118434 | HEART ATTACK? Q 8 |
| CARDIA Cohort | phv00119809 | HEART ATTACK? Q 3 |
| CARDIA Cohort | phv00121288 | MYOCARDIAL INFARCTION? |
| CARDIA Cohort | phv00121505 | MYOCARDIAL INFARCTION? |
| Cardiovascular Health Study (CHS) Cohort | phv00099015 | MYOCARDIAL INFARCTION OR HEART ATTACK |
| Cardiovascular Health Study (CHS) Cohort | phv00099450 | MI STATUS AT BASELINE |
| Cardiovascular Health Study (CHS) Cohort | phv00100292 | MI STATUS AT BASELINE |
| Cardiovascular Health Study (CHS) Cohort | phv00104040 | MYOCARDIAL INFARCTION OR HEART ATTACK |
| Cardiovascular Health Study (CHS) Cohort | phv00105872 | MI STATUS AT BASELINE |
| Cardiovascular Health Study (CHS) Cohort | phv00197703 | Prevalent heart attacks |
| CATHGEN: Genetic Mediators of Metabolic Cardiovascular Disease Risk | phv00197212 | Previous history of myocardial infarction |
| CATHGEN: Identification of Novel Genetic and Metabolomic CV Phenotypes | phv00197192 | Previous history of myocardial infarction |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00065743 | J1a. Verification of events, cardiovascular event: myocardial infarction |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00066193 | B0a. Recognition of event. Conformation of cardiovascular events. Myocardial infarction |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00030967 | Myocardial infarction |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00034353 | Patient has a history of: myocardial infarction |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00048749 | J1a. Verification of event, cardiovascular event: myocardial infarction |
| DCCT-EDIC Clinical Trial and Follow-up of Persons with Type 1 Diabetes | phv00049167 | J1a. Verification of event, cardiovascular event: myocardial infarction |
| Framingham Cohort | phv00000517 | ECG: MYOCARDIAL INFARCTION, EXAM 1 |
| Framingham Cohort | phv00000571 | ECG: MYOCARDIAL INFARCTION, EXAM 2 |
| Framingham Cohort | phv00000649 | ECG: MYOCARDIAL INFARCTION, EXAM 3 |
| Framingham Cohort | phv00000817 | ECG: MYOCARDIAL INFARCTION, EXAM 5 |
| Framingham Cohort | phv00000883 | ECG: MYOCARDIAL INFARCTION, EXAM 6 |
| Framingham Cohort | phv00001007 | ECG: MYOCARDIAL INFARCTION, EXAM 7 |
| Framingham Cohort | phv00001170 | ECG: MYOCARDIAL INFARCTION |
| Framingham Cohort | phv00001527 | ECG: MYOCARDIAL INFARCTION |
| Framingham Cohort | phv00001718 | ECG: MYOCARDIAL INFARCTION |
| Framingham Cohort | phv00001995 | ECG: MYOCARDIAL INFARCTION |
| Framingham Cohort | phv00002175 | ECG: MYOCARDIAL INFARCTION |
| Framingham Cohort | phv00002370 | ECG: MYOCARDIAL INFARCTION |
| Framingham Cohort | phv00002610 | ECG: MYOCARDIAL INFARCTION |
| Framingham Cohort | phv00003199 | ECG: MYOCARDIAL INFARCTION |
| Framingham Cohort | phv00003614 | ECG: MYOCARDIAL INFARCTION |
| Framingham Cohort | phv00007665 | MYOCARDIAL INFARCTION |
| Framingham Cohort | phv00164568 | MI status at baseline (0/1) |
| GAW16 Framingham and Simulated Data | phv00058183 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058186 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058189 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058215 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058218 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058221 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058247 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058250 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058253 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058279 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058282 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058285 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058311 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058314 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058317 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058343 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058346 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058349 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058375 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058378 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058381 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058407 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058410 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058413 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058439 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058442 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058445 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058471 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058474 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058477 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058503 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058506 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058509 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058535 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058538 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058541 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058567 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058570 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058573 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058599 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058602 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058605 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058631 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058634 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058637 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058663 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058666 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058669 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058695 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058698 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058701 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058727 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058730 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058733 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058759 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058762 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058765 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058791 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058794 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058797 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058823 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058826 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058829 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058855 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058858 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058861 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058887 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058890 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058893 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058919 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058922 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058925 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058951 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058954 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058957 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00058983 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00058986 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00058989 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059015 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059018 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059021 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059047 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059050 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059053 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059079 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059082 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059085 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059111 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059114 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059117 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059143 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059146 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059149 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059175 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059178 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059181 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059207 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059210 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059213 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059239 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059242 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059245 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059271 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059274 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059277 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059303 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059306 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059309 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059335 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059338 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059341 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059367 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059370 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059373 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059399 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059402 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059405 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059431 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059434 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059437 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059463 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059466 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059469 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059495 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059498 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059501 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059527 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059530 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059533 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059559 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059562 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059565 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059591 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059594 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059597 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059623 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059626 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059629 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059655 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059658 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059661 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059687 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059690 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059693 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059719 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059722 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059725 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059751 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059754 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059757 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059783 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059786 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059789 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059815 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059818 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059821 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059847 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059850 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059853 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059879 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059882 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059885 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059911 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059914 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059917 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059943 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059946 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059949 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00059975 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00059978 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00059981 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060007 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060010 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060013 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060039 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060042 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060045 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060071 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060074 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060077 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060103 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060106 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060109 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060135 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060138 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060141 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060167 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060170 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060173 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060199 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060202 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060205 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060231 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060234 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060237 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060263 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060266 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060269 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060295 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060298 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060301 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060327 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060330 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060333 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060359 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060362 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060365 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060391 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060394 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060397 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060423 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060426 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060429 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060455 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060458 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060461 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060487 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060490 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060493 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060519 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060522 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060525 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060551 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060554 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060557 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060583 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060586 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060589 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060615 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060618 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060621 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060647 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060650 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060653 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060679 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060682 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060685 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060711 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060714 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060717 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060743 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060746 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060749 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060775 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060778 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060781 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060807 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060810 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060813 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060839 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060842 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060845 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060871 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060874 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060877 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060903 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060906 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060909 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060935 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060938 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060941 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060967 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00060970 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00060973 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00060999 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061002 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061005 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061031 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061034 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061037 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061063 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061066 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061069 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061095 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061098 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061101 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061127 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061130 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061133 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061159 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061162 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061165 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061191 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061194 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061197 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061223 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061226 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061229 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061255 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061258 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061261 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061287 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061290 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061293 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061319 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061322 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061325 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061351 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061354 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061357 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061383 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061386 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061389 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061415 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061418 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061421 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061447 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061450 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061453 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061479 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061482 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061485 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061511 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061514 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061517 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061543 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061546 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061549 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061575 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061578 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061581 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061607 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061610 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061613 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061639 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061642 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061645 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061671 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061674 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061677 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061703 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061706 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061709 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061735 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061738 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061741 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061767 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061770 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061773 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061799 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061802 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061805 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061831 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061834 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061837 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061863 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061866 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061869 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061895 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061898 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061901 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061927 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061930 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061933 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061959 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061962 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061965 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00061991 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00061994 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00061997 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062023 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062026 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062029 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062055 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062058 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062061 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062087 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062090 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062093 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062119 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062122 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062125 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062151 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062154 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062157 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062183 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062186 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062189 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062215 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062218 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062221 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062247 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062250 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062253 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062279 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062282 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062285 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062311 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062314 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062317 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062343 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062346 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062349 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062375 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062378 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062381 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062407 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062410 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062413 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062439 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062442 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062445 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062471 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062474 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062477 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062503 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062506 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062509 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062535 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062538 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062541 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062567 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062570 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062573 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062599 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062602 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062605 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062631 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062634 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062637 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062663 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062666 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062669 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062695 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062698 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062701 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062727 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062730 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062733 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062759 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062762 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062765 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062791 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062794 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062797 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062823 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062826 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062829 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062855 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062858 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062861 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062887 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062890 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062893 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062919 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062922 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062925 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062951 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062954 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062957 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00062983 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00062986 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00062989 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063015 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063018 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063021 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063047 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063050 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063053 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063079 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063082 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063085 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063111 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063114 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063117 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063143 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063146 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063149 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063175 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063178 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063181 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063207 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063210 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063213 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063239 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063242 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063245 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063271 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063274 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063277 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063303 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063306 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063309 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063335 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063338 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063341 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063367 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063370 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063373 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063399 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063402 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063405 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063431 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063434 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063437 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063463 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063466 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063469 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063495 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063498 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063501 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063527 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063530 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063533 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063559 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063562 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063565 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063591 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063594 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063597 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063623 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063626 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063629 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063655 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063658 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063661 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063687 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063690 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063693 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063719 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063722 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063725 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063751 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063754 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063757 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063783 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063786 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063789 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063815 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063818 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063821 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063847 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063850 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063853 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063879 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063882 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063885 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063911 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063914 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063917 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063943 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063946 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063949 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00063975 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00063978 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00063981 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064007 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064010 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064013 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064039 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064042 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064045 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064071 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064074 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064077 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064103 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064106 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064109 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064135 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064138 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064141 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064167 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064170 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064173 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064199 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064202 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064205 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064231 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064234 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064237 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064263 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064266 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064269 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064295 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064298 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064301 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064327 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064330 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064333 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064359 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064362 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064365 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064391 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064394 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064397 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064423 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064426 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064429 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064455 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064458 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064461 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064487 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064490 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064493 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064519 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064522 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064525 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| GAW16 Framingham and Simulated Data | phv00064551 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 1 |
| GAW16 Framingham and Simulated Data | phv00064554 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 2 |
| GAW16 Framingham and Simulated Data | phv00064557 | 1:Myocardial infarction (MI) event, 0:No Myocardial infarction (MI) event at visit 3 |
| Genetic Epidemiology of COPD (COPDGene) | phv00159615 | Heart attack [MI] |
| GENEVA: The Atherosclerosis Risk in Communities (ARIC) Study | phv00080491 | Prevalent MI [myocardial infarction, heart attack] (composite ECG [electroencephalogram]or medical history) |
| Jackson Heart Study (JHS) Cohort | phv00124993 | 7a. Heart attack |
| Jackson Heart Study (JHS) Cohort | phv00125078 | 7a. Heart attack |
| Jackson Heart Study (JHS) Cohort | phv00125163 | 7a. Heart attack |
| Jackson Heart Study (JHS) Cohort | phv00126085 | History of MD diagnosed MI |
| Jackson Heart Study (JHS) Cohort | phv00126088 | History of MI(derived) |
| Jackson Heart Study (JHS) Cohort | phv00181224 | History of myocardial infarction (MI) recorded at first participant visit (0/1). |
| Jackson Heart Study (JHS) Cohort | phv00165331 | MI status at baseline [0=No, 1=Yes] |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087142 | HEART ATTACK |
| Multi-Ethnic Study of Atherosclerosis (MESA) Cohort | phv00087853 | MYOCARDIAL INFARCTION (MI) |
| NHGRI Genome-Wide Association Study of Venous Thromboembolism (GWAS of VTE) | phv00121850 | Ever had a heart attack (mi) |
| NHLBI ARIC Candidate Gene Association Resource (CARe) | phv00190322 | History of myocardial infarction (MI) recorded at first participant visit (0/1). |
| NHLBI GO-ESP: Early-Onset Myocardial Infarction (Broad EOMI) | phv00160316 | MI status at baseline (0/1) |
| NHLBI GO-ESP: Heart Cohorts Exome Sequencing Project (ARIC) | phv00163947 | MI status at baseline (0/1) |
| NHLBI GO-ESP: Heart Cohorts Exome Sequencing Project (CARDIA) | phv00190561 | MI status at baseline (0/1) |
| NHLBI GO-ESP: Heart Cohorts Exome Sequencing Project (ISGS) | phv00179592 | History of myocardial infarction |
| NHLBI GO-ESP: Heart Cohorts Exome Sequencing Project (MESA) | phv00178029 | MI status at baseline (0/1) |
| NHLBI GO-ESP: Women's Health Initiative Exome Sequencing Project (WHI) - WHISP | phv00161371 | HISTORY OF MI AT BASEINE |
| NIA Long Life Family Study (LLFS) | phv00163268 | Myocardial Infarction or Heart Attack diagnosed? |
| Risk Assessment of Cerebrovascular Events (RACE) Study | phv00167704 | Myocardial infarction |
| Risk Assessment of Cerebrovascular Events (RACE) Study | phv00167697 | Myocardial infarction |
| STAMPEED: Myocardial Infarction Genetics Consortium (MIGen) | phv00112168 | Myocardial Infarction (MI) status |
| STAMPEED: Myocardial Infarction Genetics Consortium (MIGen) | phv00112183 | MI status |
| The CARDIA-GENEVA Study | phv00129543 | Has/had heart attack [Exam 1]? |
| The CARDIA-GENEVA Study | phv00129548 | Had heart attack [Exam 2]? |
| The CARDIA-GENEVA Study | phv00129554 | Heart attack at Exam 3 |
| The CARDIA-GENEVA Study | phv00129559 | Heart attack [Exam 4]? |
| The CARDIA-GENEVA Study | phv00129564 | Heart attack [Exam 5]? |
| The CARDIA-GENEVA Study | phv00129569 | Heart attack [Exam 6]? |
| The CARDIA-GENEVA Study | phv00129574 | Heart attack [Exam 7]? |
| The Familial Intracranial Aneurysm Linkage Study (FIA) | phv00191133 | Myocardial infarction |
| The Genomics and Randomized Trials Network (GARNET) Vitamin Intervention Stroke Prevention (VISP) trial | phv00168770 | Myocardial infarction (reviewed endpoint) |
| Whole Exome Sequencing for Familial Intracranial Aneurysm (FIA I-II) Study | phv00193942 | Myocardial infarction |
| Whole Genome Association Study of Bipolar Disorder | phv00017032 | B1. HeartAttack, Yes/No. (Participants with European ancestry). DIGS4 |
| Whole Genome Association Study of Bipolar Disorder | phv00051936 | B1 Heart attack, Yes/No, (African American participants). DIGS4 |
| Women's Health Initiative | phv00193019 | Clinical MI Diagnosis |
Mapped dbGaP Studies
Mapping for PX040801_Myocardial_Infarction_Date
| dbGAP Study | dbGAP Variable | dbGAP Variable Description |
|---|---|---|
| Atherosclerosis Risk in Communities (ARIC) Cohort | phv00203856 | Date of myocardial infraction [MI] for cohort event-days from exam 1 [CHD Derived Variable Dictionary - Events Cohort] |
| Framingham Cohort | phv00178998 | Date of MI event |
| The Genomics and Randomized Trials Network (GARNET) Vitamin Intervention Stroke Prevention (VISP) trial | phv00180552 | Date of heart attack (if more than one, record date of first attack) |
View Image
Cite This Protocol
To cite this protocol, copy & paste the text below: