Protocol - Pulse Oximetry (Exercise)
Description
This protocol addresses exercise testing for evaluation of hypoxemia and/or desaturation.
Specific Instructions
This guideline is appropriate for pediatric, adult, and geriatric patients who are capable of following test instructions and techniques. The learning ability and communication skills of the patient being served should be taken into consideration when performing these tests. The neonatal population is not served by this guideline.
Availability
This protocol is freely available; permission not required for use.
Protocol
1.0 PROCEDURE:
Exercise testing for evaluation of hypoxemia and/or desaturation.
2.0 DESCRIPTION/DEFINITION:
Pulse oximetry provides estimates of arterial oxyhemoglobin saturation (SaO2) by utilizing selected wavelengths of light to noninvasively determine the saturation of oxyhemoglobin (SpO2).
Exercise testing may be performed to determine the degree of oxygen desaturation and/or hypoxemia that occurs on exertion. Desaturation is defined as a valid decrease in arterial oxygenation as measured by CO-oximetry saturation, (SaO2) of 2% (based on the reproducibility of HbO2 measurement at ±1%), an SaO2 < 88%, and/or a blood gas PaO2 ≤ 55 torr.
2.1 Exercise testing may also be performed to optimize titration of supplemental oxygen for the correction of hypoxemia. An SpO2 of 93% should be used as a target.
2.2 It is preferable that this procedure be performed using a method that allows quantitation of workload and heart rate achieved (as % predicted).
2.2.1 This evaluation can be incorporated into other more complex test protocols (e.g., cardiac stress testing).
2.2.2 Continuous noninvasive measurement of arterial oxyhemoglobin saturation by pulse oximetry can provide qualitative information and an approximation of oxyhemoglobin saturation, with a 4% decrease in SpO2 considered significant, but evaluation of desaturation on exertion requires analysis of arterial blood samples drawn with the subject at rest and at peak exercise.
2.3 Arterial blood specimens may be obtained by single puncture or by arterial cannulation.
2.4 This guideline is appropriate for pediatric, adult, and geriatric patients who are capable of following test instructions and techniques.
2.4.1 The learning ability and communication skills of the patient being served should be taken into consideration when performing these tests.
2.4.2 The neonatal population is not served by this guideline.
3.0 SETTINGS:
Exercise testing may be performed by trained personnel in a variety of settings including
3.1 pulmonary function laboratories
3.2 cardiopulmonary exercise laboratories
3.3 clinics
3.4 pulmonary rehabilitation facilities
3.5 physicians’ offices
4.0 INDICATIONS:
Indications for exercise testing include
4.1 the need to assess and quantify the adequacy of arterial oxyhemoglobin saturation during exercise in patients who are clinically suspected of desaturation (e.g., those who manifest dyspnea on exertion, decreased DLCO, decreased PaO2 at rest, or documented pulmonary disease);
4.2 the need to quantitate the response to therapeutic intervention (e.g., oxygen prescription, medications, smoking cessation, or to reassess the need for continued supplemental oxygen);
4.3 the need to titrate the optimal amount of supplemental oxygen to treat hypoxemia or desaturation during activity;
4.4 the need for preoperative assessment for lung resection or transplant;
4.5 the need to assess the degree of impairment for disability evaluation (e.g., pneumoconiosis, asbestosis).
5.0 CONTRAINDICATIONS:
5.1 Absolute contraindications include
5.1.1 acute electrocardiographic changes suggesting myocardial ischemia or serious cardiac dysrhythmias including bradydysrhythmias, tachydysrhythmias, sick sinus syndrome, and multifocal premature ventricular contractions (PVCs), causing symptoms or hemodynamic compromise (occasional PVCs are not a contraindication);
5.1.2 unstable angina;
5.1.3 recent myocardial infarction (within the previous 4 weeks) or myocarditis;
5.1.4 aneurysm of the heart or aorta;
5.1.5 uncontrolled systemic hypertension;
5.1.6 acute thrombophlebitis or deep venous thrombosis;
5.1.7 second- or third-degree heart block;
5.1.8 recent systemic or pulmonary embolus;
5.1.9 acute pericarditis;
5.1.10 symptomatic severe aortic stenosis;
5.1.11 uncontrolled heart failure;
5.1.12 uncontrolled or untreated asthma;
5.1.13 pulmonary edema;
5.1.14 respiratory failure;
5.1.15 acute non-cardiopulmonary disorders affected by exercise.
5.2 Relative contraindications include
5.2.1 situations in which pulse oximetry may provide invalid data (e.g., elevated HbCO, HbMet, or decreased perfusion).
5.2.2 situations in which arterial puncture and/or arterial cannulation may be contraindicated;
5.2.3 a non-compliant patient or one who is not capable of performing the test because of weakness, pain, fever, dyspnea, incoordination, or psychosis;
5.2.4 severe pulmonary hypertension (cor pulmonale);
5.2.5 known electrolyte disturbances (hypokalemia, hypomagnesemia);
5.2.6 resting diastolic blood pressure > 110 torr or resting systolic blood pressure > 200 torr;
5.2.7 neuromuscular, musculoskeletal, or rheumatoid disorders that are exacerbated by exercise;
5.2.8 uncontrolled metabolic disease (e.g., diabetes, thyrotoxicosis, or myxedema);
5.2.9 SaO2 or SpO2 < 85% on room air;
5.2.10 complicated or advanced pregnancy;
5.2.11 hypertrophic cardiomyopathy or other forms of outflow tract obstruction;
5.2.12 patient’s inability to cooperate or follow directions for testing.
6.0 PRECAUTIONS AND/OR POSSIBLE COMPLICATIONS:
6.1 Indications for immediate termination of testing include
6.1.1 electrocardiographic abnormalities (e.g., dangerous dysrhythmias, ventricular tachycardia, ST-T wave changes);
6.1.2 severe desaturation as indicated by an SaO2 ≤80% or SpO2 ≤83% (A number of pulse oximeters have been found to overestimate SpO2,) and/or a 10% fall from baseline values (Underestimation of saturation has been noted to occur with certain pulse oximeter models);
6.1.3 angina;
6.1.4 hypotensive responses;
6.1.4.1 a fall of > 20 torr in systolic pressure, occurring after the normal exercise rise;
6.1.4.2 a fall in systolic blood pressure below the pre-exercise level;
6.1.5 lightheadedness;
6.1.6 request from patient to terminate test.
6.2 Abnormal responses that may require discontinuation of exercise include
6.2.1 a rise in systolic blood pressure to > 250 torr or of diastolic pressure to > 120 torr or a rise in systolic pressure of < 20 torr from resting level;
6.2.2 mental confusion or headache;
6.2.3 cyanosis;
6.2.4 nausea or vomiting;
6.2.5 muscle cramping.
6.3 Hazards associated with arterial puncture, arterial cannulation, and pulse oximetry. Pulse oximetry is a noninvasive safe procedure, but because of device limitations, false-negative results for hypoxemia and/or false-positive results for normoxemia or hyperoxemia may lead to inappropriate treatment of the patient. Although it is rare, tissue injury may occur at the measuring site as a result of probe misuse, such as pressure sores from prolonged application or electrical shock and burns from the substitution of incompatible probes between instruments.
7.0 LIMITATIONS OF PROCEDURE/VALIDATION OF RESULTS:
7.1 Limitations of equipment
7.1.1 Because of possible limitations of pulse oximetry with exercise and at rest, measurements may read falsely low or falsely high and should be validated by comparison with baseline arterial samples analyzed by CO-oximetry.
7.1.1.1 Only a limited number of pulse oximeters have been validated with results of concurrent arterial blood gas analysis in diseased subjects under exercise conditions.
7.1.1.2 Overestimation of oxygen saturation may occur with carboxyhemoglobin saturations (> 4%).
7.1.1.3 Decreasing accuracy in SpO2 has been reported with desaturations to < 83%. This is assumed to be the result of limitations of in vivo calibration to 85% with extrapolation of the calibration curve below that value.
7.1.1.4 Decreased perfusion with cardiovascular disease, vasoconstriction, or hypothermia may result in false positive results or no valid data in some pulse oximeter models. Use of an alternative site should be evaluated (e.g., ear, finger, forehead). Alternative hand-warming methods may be used to increase circulation.
7.1.1.5 Reduced ear perfusion associated with heavy exercise has been shown to affect SpO2 in some models of pulse oximeters.
7.1.1.6 Motion artifact may appear with exercise. Some pulse oximeters are better than others at rejecting motion artifact.
7.1.1.7 Pulse oximeter response time may be inadequate to describe rapid changes in saturation.
7.1.1.8 Skin pigmentation should, in theory, not affect pulse oximeter readings, but various studies report conflicting data depending on the manufacturer and model.
7.1.1.9 Hemoglobin disorders may affect the accuracy of the pulse oximeter reading. Important underestimation of arterial saturation may result from pulse oximetry in subjects with total hemoglobin levels of ≤8 g/dL.
7.1.1.10 Pulse oximetry is less useful over the range in which large changes in PaO2 are associated with small changes in SaO2 (i.e., PaO2 ≥60 torr).
7.1.1.11 Ambient light during testing may interfere with measurements of pulse oximetry.
7.1.1.12 Exercise testing in which oxyhemoglobin saturation by pulse oximetry is the only variable measured provides limited information.
7.1.2 Limitations related to the patient:
7.1.2.1 Additional limitations common to arterial sampling and analysis under resting conditions should be considered.
7.1.2.2 Patient cooperation level or physical condition may limit the subject’s ability to exercise at a workload sufficient to evoke a response. Variables that are not adequately monitored (e.g., free walking) have limited application.
7.2 Validation of results
7.2.1 Arterial blood gas samples should be obtained at rest and at peak exercise. Samples from single arterial punctures have been shown to be equivalent to samples drawn from indwelling cannulas.
7.2.2 In the unlikely event that a single puncture at peak exercise is unsuccessful in an uncannulated patient, a sample drawn within 10-15 seconds of the termination of exercise will suffice unless analysis shows a decrease from the resting values, in which case quantitation of desaturation requires a peak exercise sample obtained by cannula.
7.2.3 Arterial blood gas results should be obtained according to the guidelines for arterial blood gas sampling and for arterial blood gas analysis.
7.2.4 Validity of pulse oximetry results is verified by comparison with the results of analysis by CO-oximetry, preferably at rest and at end of exercise.
7.2.4.1 SpO2 may be used to assess response to supplemental oxygen. If administration of supplemental oxygen does not improve a low SpO2, arterial blood analysis may be warranted.
7.2.4.2 Testing should be performed in compliance with the AARC Pulse Oximetry Clinical Practice Guideline.
7.2.4.3 Correlation between pulse oximetry heart rate and palpated pulse rate and/or electrocardiogram should be established.
7.2.4.4 Pulse oximetry with pulse waveform display may be desirable. For patients with normal adult hemoglobin, the highest accuracy and best performance is attained when the probe is attached to the patient in such a way that the arterial signal has the largest possible amplitude, which is only available with systems that yield a plethysmographic tracing.
8.0 ASSESSMENT OF NEED:
Exercise testing for evaluation of hypoxemia and/or desaturation may be indicated (see section 4.0 INDICATIONS) in the presence of
8.1 a history and physical indicators suggesting hypoxemia and/or desaturation (e.g., dyspnea, pulmonary disease);
8.2 abnormal diagnostic test results (e.g., DLCO, FEV1, resting arterial blood gases including directly measured HbO2, HbCO, and HbMet);
8.3 the need to titrate or adjust a therapy (e.g., supplemental oxygen).
9.0 ASSESSMENT OF QUALITY OF TEST AND VALIDITY OF RESULTS:
The consensus of the committee is that all diagnostic procedures should follow the quality model described in the National Committee for Clinical Laboratory Standards (NCCLS) GP26-A A Quality System Model for Health Care. (Fig. 1) The document describes a laboratory path of workflow model that incorporates all the steps of the procedure. This process begins with patient assessment and the generation of a clinical indication for testing through the application of the test results to patient care. The quality system essentials defined for all health care services provide the framework for managing the path of workflow. A continuation of this model for respiratory care services is further described in NCCLS HS4-A A Quality System Model for Respiratory Care. In both quality models the patient is the central focus.
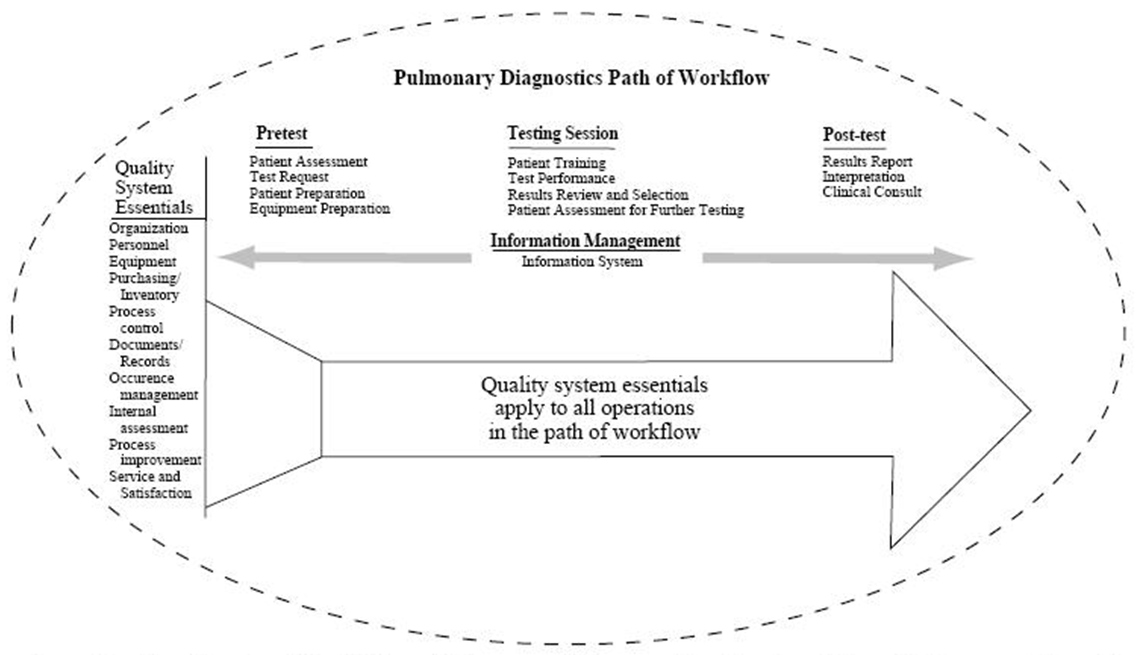
Fig 1. Structure for a Quality System Model for a Pulmonary Diagnostics Service (From Reference 55, with permission)
9.1 General considerations include:
9.1.1 As part of any quality assurance program, indicators must be developed to monitor areas addressed in the path of workflow.
9.1.2 Each laboratory should standardize procedures and demonstrate intertechnologist reliability. Test results can be considered valid only if they are derived according to and conform to established laboratory quality control, quality assurance, and monitoring protocols.
9.1.3 Documentation of results, therapeutic intervention (or lack of), and/or clinical decisions based on the exercise testing should be placed in the patient’s medical record. Report of test results should contain a statement by the technician performing the test regarding test quality (including patient understanding of directions and effort expended) and, if appropriate, which recommendations were not met.
9.1.4 The type of medications, dose, and time taken prior to testing and the results of the pretest assessment should be documented.
9.1.5 Test results should be interpreted by a physician, taking into consideration the clinical question to be answered.
9.1.6 A technologist who has not met annual competency requirements or whose competency is deemed unacceptable as documented in an occurrence report should not be allowed to participate, until he or she has received remedial instruction and has been reevaluated.
9.1.7 There must be evidence of active review of quality control, proficiency testing, and physician alert, or "panic" values, on a level commensurate with the number of tests performed.
9.2 Calibration and quality control measures specific to equipment used in exercise testing for desaturation include:
9.2.1 Calibration procedures as defined by the laboratory protocols and manufacturer’s specifications should be adhered to.
9.2.2 Treadmills and bicycle ergometers should be calibrated according to the manufacturer’s recommendations, with periodic re-verification.
9.2.3 Pulse oximeter monitors should be maintained as described under quality assurance in the manufacturer’s manual.
9.2.4 Biological controls should be tested regularly (self-testing of normal laboratory staff).
9.3 Test quality: Results of arterial blood gas analysis and/or SpO2 should confirm or rule out oxygen desaturation during exercise to validate the patient’s clinical condition.
9.4 Test results: The exercise should have a symptom-limited or physiologic end point documented (e.g., heart rate or onset of dyspnea).
10.0 RESOURCES:
10.1 Equipment
10.1.1 Treadmill, cycle ergometer, or equivalent equipment, adaptable to patients who may be severely limited (e.g., low-speed treadmill, low-watt ergometer, arm crank ergometer). Other forms of exercise may be utilized (stair climbing, step test, timed walking); however, such modes do not eliminate the necessity for adequate monitoring as described in Sections 7 and 9 and the necessity for adequate documentation of procedure and patient response.
10.1.2 Arterial blood sampling equipment for single puncture or arterial cannulation and analyzers that have been properly calibrated and for which multilevel controls indicate proper function. (Note from the Working Group: Only required if arterial blood gas validation will be performed)
10.1.3 Pulse oximeter monitor and related accessories.
10.1.4 Electrocardiographic monitor with the capacity to monitor heart rate to a predicted maximum and accurately display cardiac rhythm during exercise. (Multiple leads are preferred.)
10.1.5 Resuscitation equipment including oxygen with various delivery devices, such as nasal cannula and mask.
10.1.6 An easily accessible cardiac arrest cart and defibrillator with resuscitation equipment.
10.1.7 Blood pressure monitoring device, manual or automatic. (If an automated system is used, a manual blood pressure cuff and stethoscope should be available as a backup.)
10.1.8 Visual aids (e.g., Borg scales for dyspnea and fatigue) that are large, easy to read, and in clear view.
10.1.9 Blood gas sampling and analysis equipment.
10.2 Background history and data
10.2.1 Results of appropriate baseline diagnostic tests and patient history (e.g., electrocardiogram, chest radiograph, and pulmonary function test results) should be available.
10.2.2 The need for written consent should be determined within the specific institution.
10.2.3 A list of the patient’s current medications and any pharmacologic allergies should be included.
10.3 Personnel
10.3.1 The presence of a physician trained in exercise testing may be required depending on patient condition and hospital policy.
10.3.2 Personnel administering the test should possess experience and knowledge in exercise physiology and testing, including arterial blood gas sampling and analysis; cardiopulmonary resuscitation (certified in Basic Cardiac Life Support, or BCLS. Qualification in Advanced Cardiac Life Support, or ACLS, is recommended); ECG abnormality recognition; oxygen therapy; blood pressure monitoring; and application and limitations of pulse oximeters. Training and demonstrated competency must be documented for all testing personnel.
10.3.3 Testing personnel should have the knowledge and skills to respond to adverse situations with the patient and to know when cessation of further testing is indicated (versus coaching the patient to continue).
11.0 MONITORING:
11.1 Recommended monitoring of patient during testing
11.1.1 Electrocardiograph with strip recorder, preferably screened in real-time to check for displaced leads
11.1.2 Oxygen delivery devices with documented FDO2
11.1.3 Physical assessment (chest pain, leg cramps, color, perceived exertion, dyspnea)
11.1.4 Respiratory rate
11.1.5 Patient cooperation and effort level
11.1.6 Borg, modified Borg, or visual analog dyspnea or symptom scales
11.1.7 Blood gas sampling using site and technique consistent with the AARC Clinical Practice Guideline for blood gas sampling, and NCCLS Guidelines.
11.1.8 Continuous monitoring of oxygenation status (SpO2)
11.1.9 Heart rate, rhythm, and ST-T wave changes
11.1.10 Blood pressure.
11.2 Recommended equipment monitoring during testing: Pulse waveforms of SpO2 and/or SaO2 should be analyzed to assure adequate signal acquisition for reliable readings.
12.0 FREQUENCY:
The frequency of testing depends on the patient’s clinical condition and the need for changes in therapy. Exercise may be repeated for certification of supplemental oxygen needs.
13.0 INFECTION CONTROL:
13.1 The staff, supervisors, and physician-directors associated with the pulmonary laboratory should be conversant with the "Guideline for Isolation Precautions in Hospitals" made by the Centers for Disease Control and the Hospital Infection Control Practices Advisory Committee (HICPAC), and develop and implement policies and procedures for the laboratory that comply with its recommendations for standard precautions and transmission-based precautions.
13.2. The laboratory’s manager and its medical director should maintain communication and cooperation with the institution’s infection control service and the personnel health service to help assure consistency and thoroughness in complying with the institution’s policies related to immunizations, post-exposure prophylaxis, and job- and community-related illnesses and exposures.
13.3 Primary considerations include:
13.3.1 adequate hand washing,
13.3.2 provision of prescribed ventilation with adequate air exchanges,
13.3.3 careful handling and thorough cleaning and processing of equipment.
Procedure-specific considerations include:
13.3.3.1 disposable items are for single patient use;
13.3.3.2 disposable electrodes should be used for electrocardiographic monitoring with standard precautions observed during patient skin preparation. Cables and equipment that touch the patient should be wiped down with a disinfectant after each use;
13.3.3.3 reusable pulse oximeter probes should be cleaned between patient use, following the manufacturer’s guidelines.
13.3.4 the exercise of particular care in scheduling and interfacing with the patient in whom a diagnosis has not been established.
14.0 AGE-SPECIFIC ISSUES
14.1 This guideline does not apply to the neonatal population.
14.2 This CPG document applies to pediatric, adolescent, adult, and geriatric populations.
14.3 Test instructions and techniques should be given in a manner that takes into consideration the learning ability, communication skills, and age of the patient being served.
Personnel and Training Required
The presence of a physician trained in exercise testing may be required depending on patient condition and hospital policy. Personnel administering the test should possess experience and knowledge in exercise physiology and testing, including arterial blood gas sampling and analysis, cardiopulmonary resuscitation (certified in Basic Cardiac Life Support, or BCLS; Qualification in Advanced Cardiac Life Support, or ACLS, is recommended), electrocardiogram (ECG) abnormality recognition, oxygen therapy, blood pressure monitoring, and application and limitations of pulse oximeters. Training and demonstrated competency must be documented for all testing personnel. Testing personnel should have the knowledge and skills to respond to adverse situations with the patient and to know when cessation of further testing is indicated (versus coaching the patient to continue).
Equipment Needs
- Treadmill, cycle ergometer, or equivalent equipment, adaptable to patients who may be severely limited (e.g., low-speed treadmill, low-watt ergometer, arm crank ergometer). Other forms of exercise may be used (stair climbing, step test, timed walking); however, such modes do not eliminate the need for adequate monitoring as described in protocol Sections 7 and 9 and the need for adequate documentation of procedure and patient response.
- Arterial blood sampling equipment for single puncture or arterial cannulation and analyzers that have been properly calibrated and for which multilevel controls indicate proper function (Note: Only required if arterial blood gas validation will be performed).
- Pulse oximeter monitor and related accessories.
- Electrocardiographic monitor with the capacity to monitor heart rate to a predicted maximum and accurately display cardiac rhythm during exercise (Multiple leads are preferred).
- Resuscitation equipment, including oxygen with various delivery devices, such as nasal cannula and mask.
- An easily accessible cardiac arrest cart and defibrillator with resuscitation equipment.
- Blood pressure monitoring device, manual or automatic (If an automated system is used, a manual blood pressure cuff and stethoscope should be available as a backup).
- Visual aids (e.g., Borg scales for dyspnea and fatigue) that are large, easy to read, and in clear view.
Blood gas sampling and analysis equipment (Note: Only required if arterial blood gas validation will be performed).
Requirements
| Requirement Category | Required |
|---|---|
| Major equipment | Yes |
| Specialized training | Yes |
| Specialized requirements for biospecimen collection | Yes |
| Average time of greater than 15 minutes in an unaffected individual | Yes |
Mode of Administration
Physical Measurement
Lifestage
Child, Adolescent, Adult
Participants
Appropriate for pediatric to geriatric populations
Selection Rationale
The protocol was selected because it is supported by the American Association of Respiratory Care (AARC). Respiratory care therapists administer this testing in most clinical circumstances.
Language
Chinese, English
Standards
| Standard | Name | ID | Source |
|---|---|---|---|
| Logical Observation Identifiers Names and Codes (LOINC) | Resp pulse ox exercise proto | 62628-3 | LOINC |
| caDSR Form | PhenX PX091001 - Pulse Oximetry Exercise | 5969115 | caDSR Form |
Derived Variables
None
Process and Review
Expert Review Panel #6 (ERP 6) reviewed the measures in the Respiratory domain.
Guidance from ERP 6 includes the following:
• No significant changes to measure
Back-compatible: no changes to Data Dictionary
Protocol Name from Source
AARC Clinical Practice Guideline: Exercise Testing for Evaluation of Hypoxemia and/or Desaturation: revision & update. Resp Care, 2001
Source
AARC Clinical Practice Guideline: Exercise Testing for Evaluation of Hypoxemia and/or Desaturation: 2001 revision & update. Respiratory Care, 46(5), 514-522.
General References
Stoller, J. K., Aboussouan, L. S., Kanner, R. E., Wilson, L. A., Diaz, P., & Wise, R.; LOTT Research Group. (2015). Characteristics of Alpha-1 Antitrypsin-Deficient Individuals in the Long-term Oxygen Treatment Trial and Comparison with Other Subjects with Chronic Obstructive Pulmonary Disease. Annals of the American Thoracic Society, 12(12),1796-1804.
Protocol ID
91001
Variables
Export Variables| Variable Name | Variable ID | Variable Description | dbGaP Mapping | |
|---|---|---|---|---|
| PX091001_Contraindications | ||||
| PX091001140000 | Contraindications for test | N/A | ||
| PX091001_Indication_For_Test | ||||
| PX091001130000 | Clinical or Research Indication for Test | N/A | ||
| PX091001_Medication 1_Date_Taken | ||||
| PX091001040000 | Time and date last taken | Variable Mapping | ||
| PX091001_Medication 1_Time_Taken | ||||
| PX091001030000 | Time and date last taken | Variable Mapping | ||
| PX091001_Medication1_Dose | ||||
| PX091001020000 | Medication Dose 1 | Variable Mapping | ||
| PX091001_Medication1_Name | ||||
| PX091001010000 | Medication Name 1 | Variable Mapping | ||
| PX091001_Medication2_Date_Taken | ||||
| PX091001080000 | Time and date last taken | N/A | ||
| PX091001_Medication2_Dose | ||||
| PX091001060000 | Medication Dose 2 | N/A | ||
| PX091001_Medication2_Name | ||||
| PX091001050000 | Medication Name 2 | N/A | ||
| PX091001_Medication2_Time_Taken | ||||
| PX091001070000 | Time and date last taken | N/A | ||
| PX091001_Medication3_Date_Taken | ||||
| PX091001120000 | Time and date last taken | N/A | ||
| PX091001_Medication3_Dose | ||||
| PX091001100000 | Medication Dose 3 | N/A | ||
| PX091001_Medication3_Name | ||||
| PX091001090000 | Medication Name 3 | N/A | ||
| PX091001_Medication3_Time_Taken | ||||
| PX091001110000 | Time and date last taken | N/A | ||
| PX091001_SAO2 | ||||
| PX091001160000 | Arterial oxyhemoglobin saturation (SaO2) | N/A | ||
| PX091001_SPO2 | ||||
| PX091001150000 | Saturation of oxyhemoglobin (SpO2) | Variable Mapping | ||
Measure Name
Pulse Oximetry (Exercise)
Release Date
January 29, 2010
Definition
This measure addresses exercise testing for evaluation of hypoxemia and/or desaturation that occurs on exertion.
Purpose
Exercise testing is used to assess and quantify the adequacy of arterial oxyhemoglobin saturation during exercise in patients who are clinically suspected of desaturation (e.g., those who manifest dyspnea on exertion, decreased DLCO, decreased PaO2 at rest, or documented pulmonary disease); to quantitate the response to therapeutic intervention (e.g., oxygen prescription, medications, smoking cessation, or to reassess the need for continued supplemental oxygen); to titrate the optimal amount of supplemental oxygen to treat hypoxemia or desaturation during activity; for preoperative assessment for lung resection or transplant; and to assess the degree of impairment for disability evaluation (e.g., pneumoconiosis, asbestosis).
Keywords
Respiratory, exercise, oxygen saturation, oxyhemoglobin, hypoxemia, desaturation, American Association of Respiratory Care, AARC
Measure Protocols
| Protocol ID | Protocol Name |
|---|---|
| 91001 | Pulse Oximetry (Exercise) |